Carbohydrate Reactions: A Comprehensive Guide
Master carbohydrate chemistry with clear explanations of oxidation, reduction, dehydration, Tollen’s test, and step-up/step-down reactions—ideal for students and researchers.
BIOTECHNOLOGY
Dr. Mainak Mukhopadhyay
9/10/202511 min read
Introduction
Among the most prevalent groups of biomolecules found in nature are carbohydrates. In biological systems, they function as the main suppliers of energy, building blocks, and signaling molecules. Carbohydrates are chemically defined as polyhydroxy aldehydes, ketones, and their derivatives. The combination of a carbonyl group and several hydroxyl groups causes carbohydrates to go through a wide range of chemical reactions. Exploring the structural diversity, biological significance, and industrial use of these reactions requires an understanding of them.
We will examine the key chemical reactions involving carbohydrates in this article, including examples, explanations, and context.
Please go through the "Carbohydrate Stereochemistry: A Complete Guide for Students" before starting the article.
1. Reactions of the Carbonyl Group
(a) Reduction Reaction
Carbohydrates contain a carbonyl group (–C=O) in the form of either an aldehyde group (–CHO) in aldoses or a keto group (–C=O) in ketoses. This carbonyl group can undergo reduction to form polyhydroxy alcohols, also known as sugar alcohols (alditols).
General Reaction:
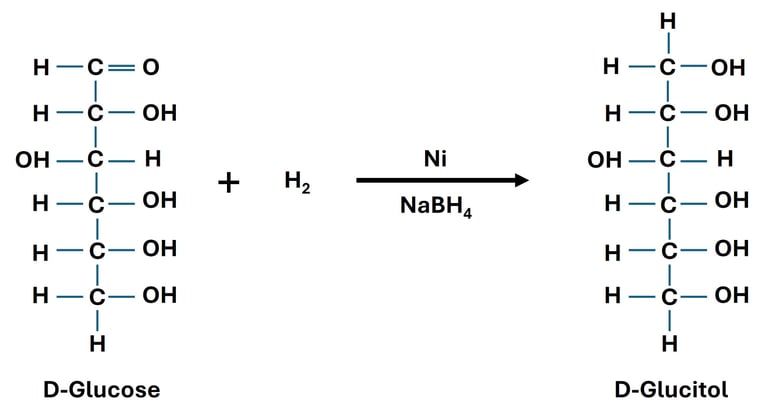
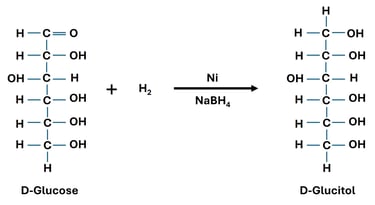
Common Reducing Agents:
Catalytic hydrogenation (H₂/Ni, H₂/Pd, H₂/Pt)
Chemical reducing agents such as sodium borohydride (NaBH₄)
Examples of Important Reactions
Glucose → Sorbitol
Sorbitol is widely used in the food and pharmaceutical industries as a sweetener, humectant, and laxative.Mannose → Mannitol
Mannitol is used as a diuretic and in reducing intracranial pressure in medical treatments.Fructose → Sorbitol + Mannitol
Explains why fructose reduction gives two sugar alcohols.
Significance of Reduction Reactions
Industrial Importance: Sorbitol and mannitol are used as artificial sweeteners in sugar-free products.
Medical Applications: Mannitol is administered to reduce brain swelling and treat glaucoma.
Biological Role: Polyols like ribitol form structural parts of coenzymes (e.g., ribitol in riboflavin).
Research Use: Formation of sugar alcohols helps in confirming the structure of the parent carbohydrate.
(b) Oxidation Reaction
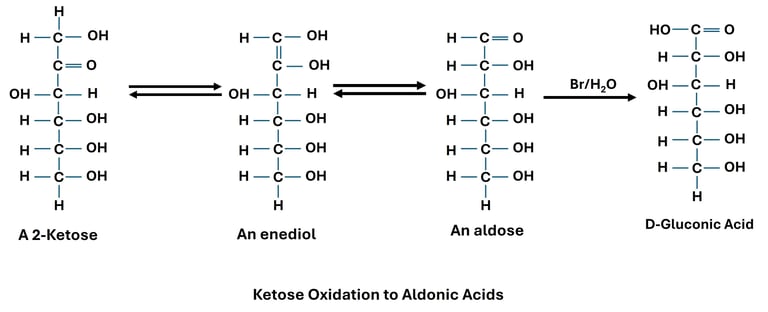
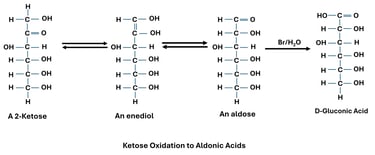
Oxidation of Aldehyde Group (Aldoses → Aldonic Acids)
Reaction: The aldehyde group (–CHO) at carbon-1 of an aldose is oxidized to a carboxylic acid (–COOH).
Reagent: Mild oxidizing agents such as Bromine water.
Significance: Aldonic acids are used in food and pharmaceutical industries.
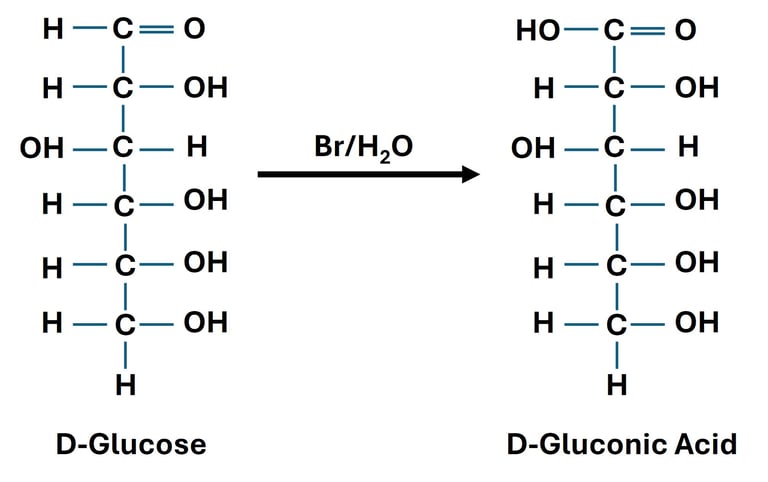
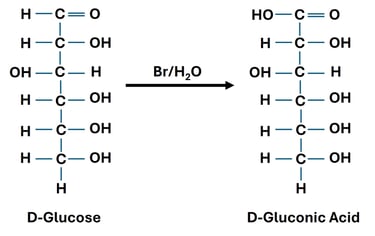
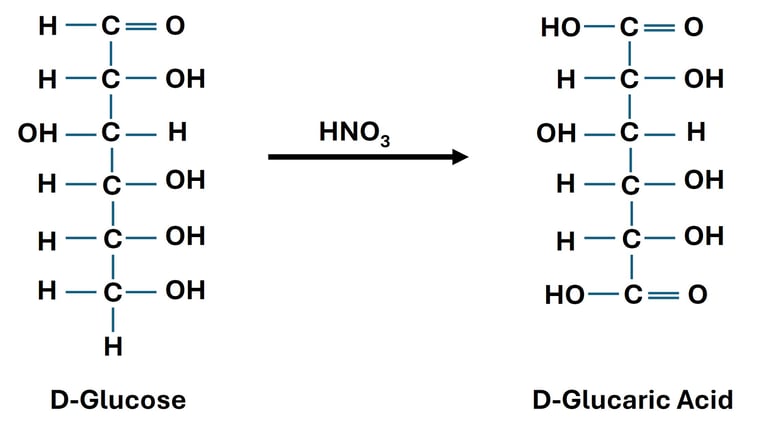
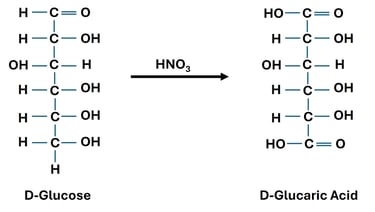
Oxidation of Both Terminal Groups (→ Aldaric Acids)
Reaction: Both the aldehyde group at carbon-1 and the primary alcohol group at carbon-6 of an aldose are oxidized to carboxylic acids.
Reagent: Strong oxidizing agent such as Nitric acid (HNO₃).
Significance: Used for structural determination of monosaccharides, since glucose and galactose yield different aldaric acids.
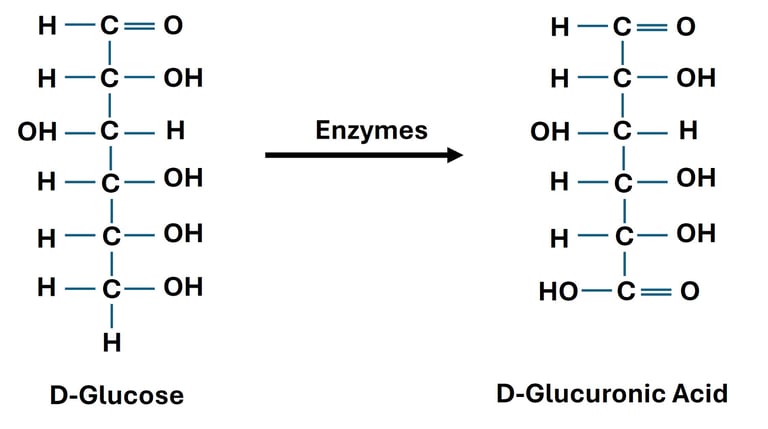
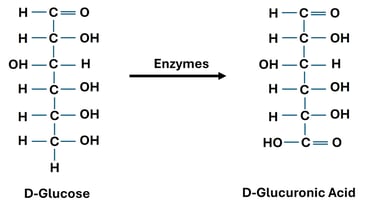
Oxidation of Primary Alcohol Group (→ Uronic Acids)
Reaction: The terminal primary alcohol group (–CH₂OH) of a monosaccharide is oxidized to a carboxylic acid, while the aldehyde group remains unchanged.
Reagent: Enzymatic or mild oxidation (e.g., biological systems).
Significance: Uronic acids play a role in detoxification of drugs in the liver (via glucuronidation).
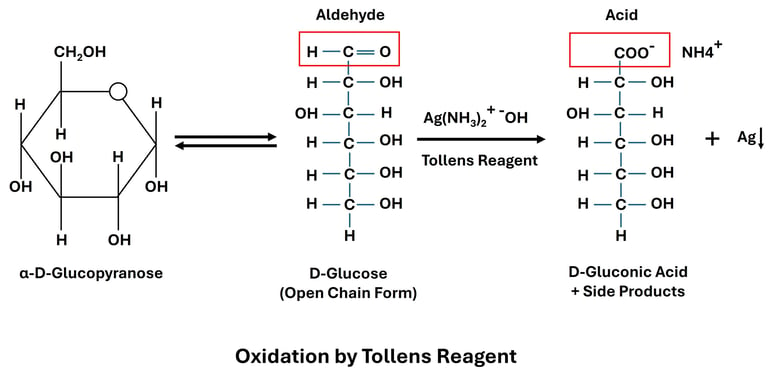
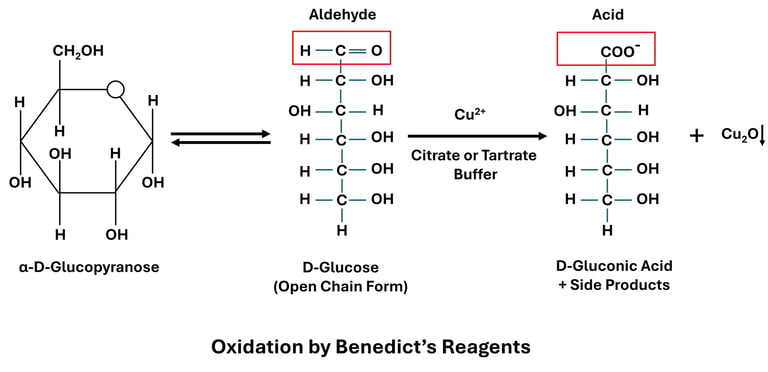
Oxidation by Tollen’s and Benedict’s Reagents
Reaction: Reducing sugars oxidize Tollen’s reagent (Ag⁺) to metallic silver and Benedict’s reagent (Cu²⁺) to cuprous oxide (Cu₂O).
Examples:
Glucose + Tollen’s reagent → Silver mirror
Glucose + Benedict’s reagent → Red precipitate of Cu₂O
Significance: These tests are used to detect reducing sugars in laboratory and clinical diagnosis (e.g., glucose in urine for diabetes).
Oxidation of Ketoses
Normally, ketones are resistant to oxidation. However, in alkaline medium, ketoses undergo enolization to form aldose intermediates, which can then be oxidized.
Example:
Fructose (a ketose) gives a positive Fehling’s test due to conversion into glucose and mannose in alkaline solution.
Biological Significance of Oxidation
Energy Metabolism: Oxidation of glucose via glycolysis and the TCA cycle produces ATP.
Detoxification: Glucuronic acid derivatives help in excreting drugs, hormones, and toxins.
Structural Role: Uronic acids are components of polysaccharides like hyaluronic acid and heparin.
2. Reactions of Hydroxyl Groups
As polyhydroxy aldehydes or ketones, carbohydrates have a carbonyl group and many hydroxyl groups (-OH). Because of their high reactivity, the hydroxyl groups play a significant role in structural analysis as well as the creation of carbohydrate derivatives.
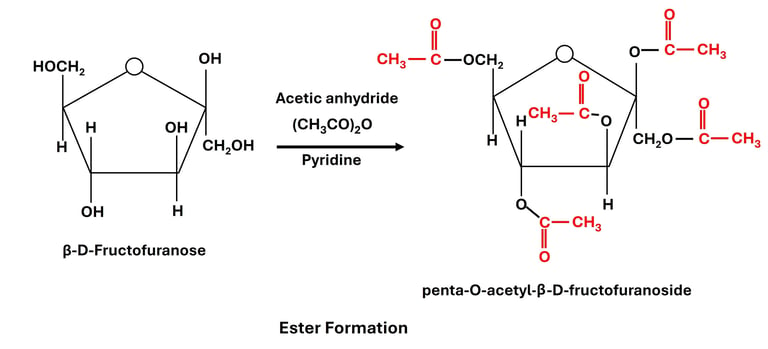
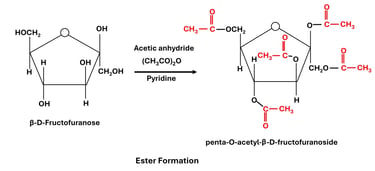
(a) Esterification of Hydroxyl Groups
Reaction: Carbohydrate hydroxyl groups react with acids or acid anhydrides to form esters.
All hydroxyl groups of glucose can be acetylated, producing penta-acetate derivatives.
Significance:
Used to study carbohydrate structures (since esterification "blocks" hydroxyl groups).
Acetylated sugars are more stable and soluble in organic solvents, useful in chemical synthesis.
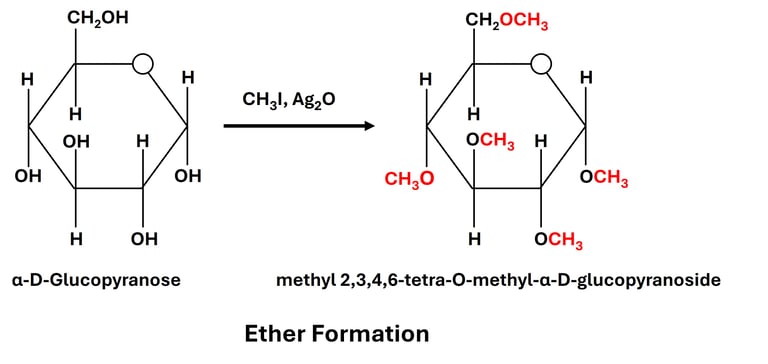
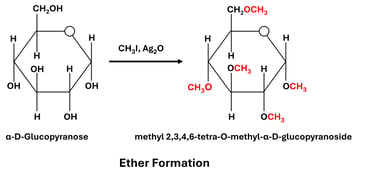
(b) Ether Formation
Reaction: Hydroxyl groups react with alkyl halides in the presence of a base to form ethers.
Significance:
Ether derivatives help in protecting hydroxyl groups during organic synthesis.
Used to identify which hydroxyl groups are free or bound in polysaccharides.
3. Osazone Formation
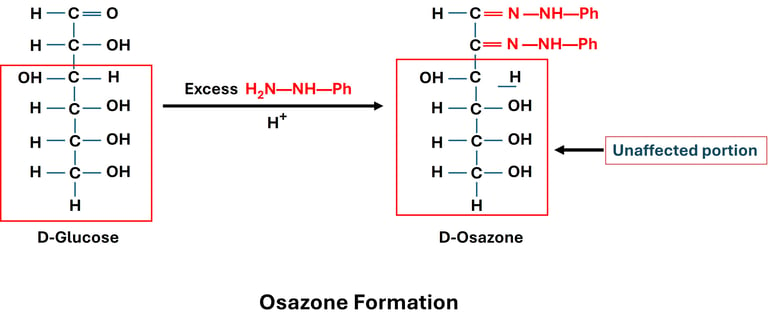
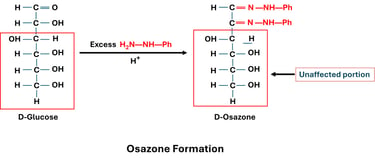
1. Principle of Osazone Formation
Reducing sugars (those with a free carbonyl group) react with phenylhydrazine under heating.
The reaction involves the carbonyl group (C1 in aldoses, C2 in ketoses) and the hydroxyl group of the adjacent carbon (C2 in aldoses).
The end product is a crystalline derivative called an osazone.
2. Reaction Steps
First Reaction (Hydrazone Formation):
The carbonyl group of the sugar reacts with one molecule of phenylhydrazine → forms sugar hydrazone.
Second Reaction (Oxidation at C2):
A second molecule of phenylhydrazine oxidizes the hydroxyl group at C2 → converts it into a carbonyl group.
Third Reaction (Osazone Formation):
The new carbonyl group reacts with a third molecule of phenylhydrazine → forms osazone.
4. Ruff Degradation
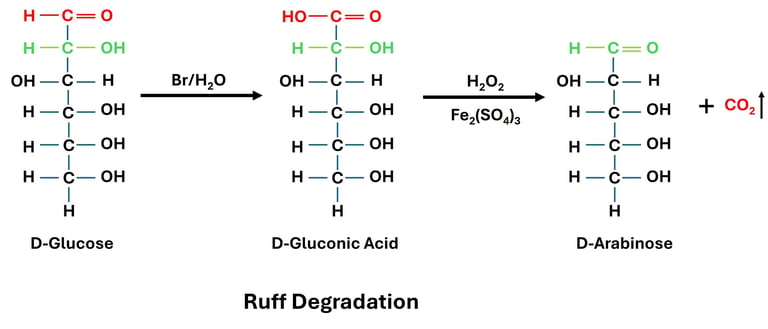
A step-down process, Ruff degradation is a classical reaction in carbohydrate chemistry in which an aldose's carbon chain is shortened by one carbon atom. Otto Ruff created the reaction, which was a crucial instrument in the early investigations of sugar structures. It involves oxidation followed by decarboxylation and is particularly applicable to aldoses.
In order to create the appropriate aldonic acid (gluconic acid in the case of glucose), an aldose, such as glucose, is first oxidized using bromine water. After that, hydrogen peroxide is applied to this aldonic acid while ferric ions (Fe³⁺) act as a catalyst. Oxidative decarboxylation takes place in this process, releasing carbon dioxide and creating an aldose that has one less carbon. For example, Ruff degradation transforms the six-carbon sugar D-glucose into the five-carbon sugar D-arabinose.
Ruff degradation is significant because it has been used historically to clarify the structure of carbohydrates by comparing higher and lower homologs, which allowed researchers to infer sugar structures. Additionally, it was a supplementary technique to the Kiliani–Fischer synthesis, which lengthens rather than shortens the aldose chain. Ruff degradation has been largely superseded by contemporary analytical and synthetic methods, but because of its conceptual and historical significance, it is still a crucial reaction in the teaching of carbohydrate chemistry.
5. Kiliani-Fischer Synthesis
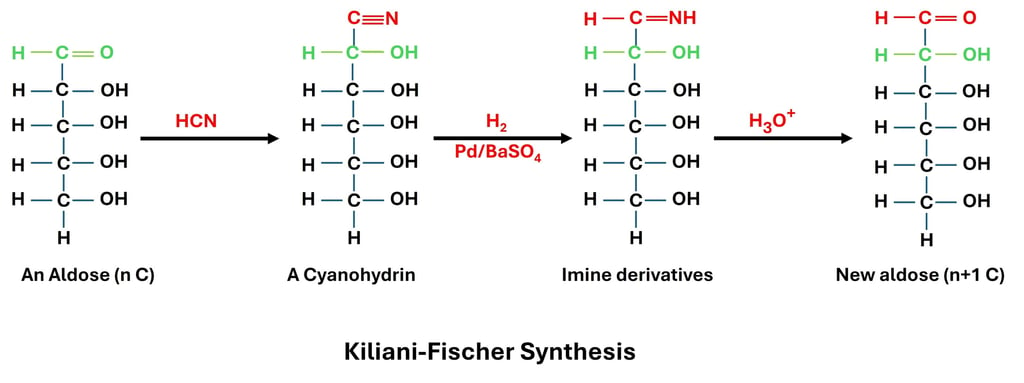
In carbohydrate chemistry, the Kiliani–Fischer synthesis is a traditional reaction that enables aldose sugars to undergo step-up extension by one carbon atom. It was created by Emil Fischer and Hermann Kiliani and was an essential technique for examining and contrasting the structures of monosaccharides. Because it allows scientists to transform a known aldose into its higher homolog and discover structural links amongst sugars, this reaction is especially significant.
When an aldose and hydrogen cyanide (HCN) combine to generate a cyanohydrin, the process starts. After that, the cyanohydrin is hydrolyzed to produce an aldonic acid, which is lactonized. The lactone is changed into a new aldose with one extra carbon atom when it is reduced with sodium amalgam or catalytically hydrogenated. For instance, the five-carbon aldose D-arabinose can be changed into the six-carbon aldoses D-glucose and D-mannose. This happens as a result of the reaction producing two potential stereoisomers by introducing a new stereocenter at the carbon next to the original aldehyde group.
Because it enabled chemists to construct homologous sequences of sugars and analyze their chemical properties, the Kiliani–Fischer synthesis has historically been crucial in figuring out the configuration of monosaccharides. The reaction is still very important in teaching since it shows how carbon chain elongation creates new stereoisomers, even though it has been mostly supplanted by contemporary synthetic techniques. The Kiliani–Fischer synthesis lengthens aldose chains, whereas Ruff degradation shortens them; hence, the two reactions are complimentary in the study of carbohydrate chemistry.
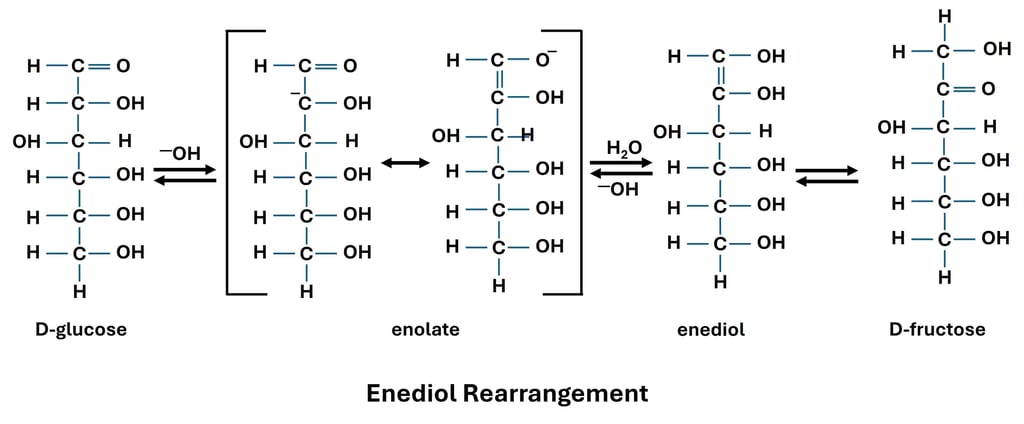
6. Enediol Rearrangement/ Enolization
In carbohydrate chemistry, enolization is a crucial chemical process where a monosaccharide interconverts between its aldose and ketose forms by forming an intermediate enediol. The hydrogen atom on the carbon next to the carbonyl group (α-hydrogen) becomes slightly acidic when a monosaccharide is exposed to a mildly alkaline media, and a base can remove it. An enolate ion is created as a result, and it is subsequently protonated to create an enediol, which is a molecule with two carbons joined by a double bond and hydroxyl groups.
Because of its chemical reactivity, this enediol intermediate can change from an aldose to a ketose or the other way around. D-glucose, for instance, can isomerize into D-fructose, a ketose, and D-mannose, an epimer at C2, in an alkaline solution. The Lobry de Bruyn–van Ekenstein transition is the name given to this phenomenon. Even though fructose is a ketose, enolization allows it to function as a reducing sugar and produce favorable Fehling's and Benedict's test results after being transformed into glucose or mannose.
Both analytically and physiologically, enolization is important. Similar enediol intermediates are produced in live systems during the isomerization of glucose-6-phosphate to fructose-6-phosphate by enzymes during glycolysis and gluconeogenesis. In the lab, enolization contributes to several side reactions, including the browning or caramelization of sugars in alkaline environments, and explains the reducing tendency of ketoses. Students who comprehend enolization are better able to relate the reactivity of carbohydrates to metabolic processes and analytical procedures in biochemistry.
7. Epimerization
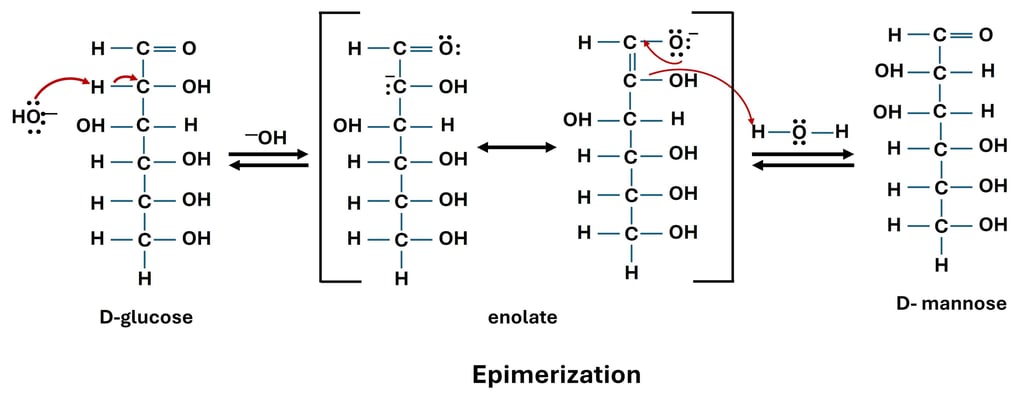
The chemical process of epimerization involves altering the structure around a single stereogenic carbon atom to change one stereoisomer (epimer) of a sugar into another. Epimers are sugars found in carbohydrates that differ only at a single carbon center, with the exception of the anomeric carbon (C1 in aldoses). In contrast to D-glucose and D-galactose, which are C4 epimers, D-glucose and D-mannose are C2 epimers. As a result, epimerization is a kind of stereoisomeric transformation and a crucial reaction for comprehending sugar interconversions.
Under alkaline conditions, epimerization can be accomplished in the lab, frequently with the use of an enediol intermediate. An enolate that reorganizes into an enediol is created when the base eliminates the α-hydrogen next to the carbonyl carbon. Depending on whether face of the double bond is protonated, this enediol can either regenerate the original sugar or form its epimer when it is reprotonated. For instance, a combination of D-glucose, D-mannose (C2 epimer), and D-fructose is produced when D-glucose is treated with diluted alkali.
Certain epimerase enzymes catalyze the process of epimerization, which also takes place in biological systems. In the Leloir pathway of galactose metabolism, for example, UDP-glucose-4-epimerase transforms UDP-glucose into UDP-galactose, which is necessary for the synthesis of lactose in mammals. Because it clarifies how the body interconverts various monosaccharides and preserves the structural diversity of carbohydrates, an understanding of epimerization is essential.
8. Conclusion
Oxidation Reactions – Convert aldehyde groups to acids (e.g., Benedict’s, Tollen’s, Bromine water tests).
Reduction Reactions – Convert carbonyl groups to alcohols (e.g., formation of alditols like sorbitol).
Reactions of Hydroxyl Groups – Include esterification, ether formation, and glycoside formation.
Osazone Formation – Forms characteristic needle-shaped crystals for sugar identification.
Ruff Degradation – Step-down reaction shortening aldose chain by one carbon.
Kiliani–Fischer Synthesis – Step-up reaction lengthening aldose chain by one carbon.
Enolization – Interconversion of aldoses and ketoses via enediol intermediate (Lobry de Bruyn–van Ekenstein transformation).
Epimerization – Conversion of one epimer to another (e.g., glucose ⇌ mannose).
Biological Importance – Many of these reactions occur in metabolism, diagnostics, and sugar biosynthesis.
9. FAQs
1. What are the most important reactions of carbohydrates?
The key reactions include oxidation, reduction, enolization, epimerization, osazone formation, glycoside formation, and chain-length modification reactions like Ruff degradation (step-down) and Kiliani–Fischer synthesis (step-up).
2. Why are oxidation reactions important in carbohydrate chemistry?
Oxidation reactions help distinguish aldoses from ketoses and identify reducing sugars. They are widely used in diagnostic tests such as Benedict’s and Tollen’s tests to detect glucose in biological samples.
3. What is the difference between Ruff degradation and Kiliani–Fischer synthesis?
Ruff degradation shortens the carbon chain of an aldose by one carbon (step-down reaction), whereas Kiliani–Fischer synthesis lengthens the carbon chain by one carbon (step-up reaction).
4. Why is osazone formation used in sugar identification?
Osazone formation produces characteristic crystalline shapes that help differentiate sugars like glucose, fructose, maltose, and lactose under a microscope.
5. What is enolization and why is it important?
Enolization is the interconversion of aldoses and ketoses through an enediol intermediate. It explains why ketoses act as reducing sugars and plays a key role in metabolic pathways such as glycolysis.
6. What is epimerization in carbohydrates?
Epimerization is the conversion of one sugar epimer to another by changing the configuration at a single carbon atom (e.g., glucose ⇌ mannose).
7. Do these reactions occur inside living organisms?
Yes! Enolization, epimerization, oxidation, and reduction reactions occur naturally in metabolic pathways like glycolysis, gluconeogenesis, and the pentose phosphate pathway.
8. How are these reactions useful in clinical diagnosis?
Tests like Benedict’s and Tollen’s rely on oxidation of reducing sugars to detect glucose in urine (a key test for diabetes).
9. Can ketoses give a positive result in Benedict’s test?
Yes, ketoses like fructose can give a positive result after enolization, which converts them into aldose forms under alkaline conditions.
10. Why should students learn these reactions?
Understanding carbohydrate reactions helps students connect organic chemistry with biochemistry, making it easier to grasp metabolic pathways, diagnostics, and industrial applications.
9. Analytical & Critical Thinking Questions
A. Analytical / Practice Questions
Differentiate between reducing and non-reducing sugars with suitable examples.
Write the reaction mechanism for the oxidation of glucose with Tollen’s reagent.
Explain how Ruff degradation converts D-glucose into D-arabinose.
Describe the steps involved in Kiliani–Fischer synthesis using D-arabinose as a starting material.
Illustrate enolization of D-glucose and show how it forms D-fructose and D-mannose.
Explain why fructose gives a positive result with Fehling’s solution even though it is a ketose.
Draw the osazone crystals formed by glucose, fructose, and maltose and explain why they look similar.
Compare the oxidation of glucose using bromine water vs. nitric acid (products formed).
Write short notes on glycoside formation and its biological importance.
Show with structures how epimerization interconverts D-glucose and D-mannose.
B. Critical Thinking Questions
If two unknown sugar samples form identical osazone crystals, can we conclude they are the same sugar? Why or why not?
How could you use a combination of Ruff degradation and Kiliani–Fischer synthesis to determine the configuration of an unknown aldose?
Why is the enediol intermediate so crucial in carbohydrate chemistry? Discuss its reactivity.
How do mild and strong oxidizing agents give different products with the same aldose?
Consider that a new sugar gives a positive Tollen’s test but does not form an osazone. What does this indicate about its structure?
Why does epimerization lead to a mixture of products instead of a single epimer?
In a diagnostic lab, why might it be important to distinguish between aldoses and ketoses even if both are reducing sugars?
What would be the effect of performing osazone formation at too high a temperature or for too long?
Could a non-reducing sugar ever give a positive Benedict’s test under special conditions? Justify your answer.
Discuss the significance of understanding carbohydrate reactions for drug design and glycobiology research.
Author Details
Dr. Mainak Mukhopadhyay
Associate Professor
Department of Biosciences
JIS University, Kolkata
(Ph.D. from Indian Institute of Technology Kharagpur, 2014)
Google Scholar Profile: https://scholar.google.com/citations?user=7mKAs4UAAAAJ&hl=en
Explore
Stay updated with biotech insights and research.
Connect
Discover
m.mukhopadhyay1212@gmail.com
+91-8777294577
© 2025. All rights reserved.